R-Water LLC, a Texas-based WOSB, manufactures a wall-mount device that enables healthcare facilities to generate a non-toxic, one-step healthcare grade cleaner disinfectant, TK60, and a multi-surface cleaner, FC+, on-site, using only electricity, softened water and pure salt.
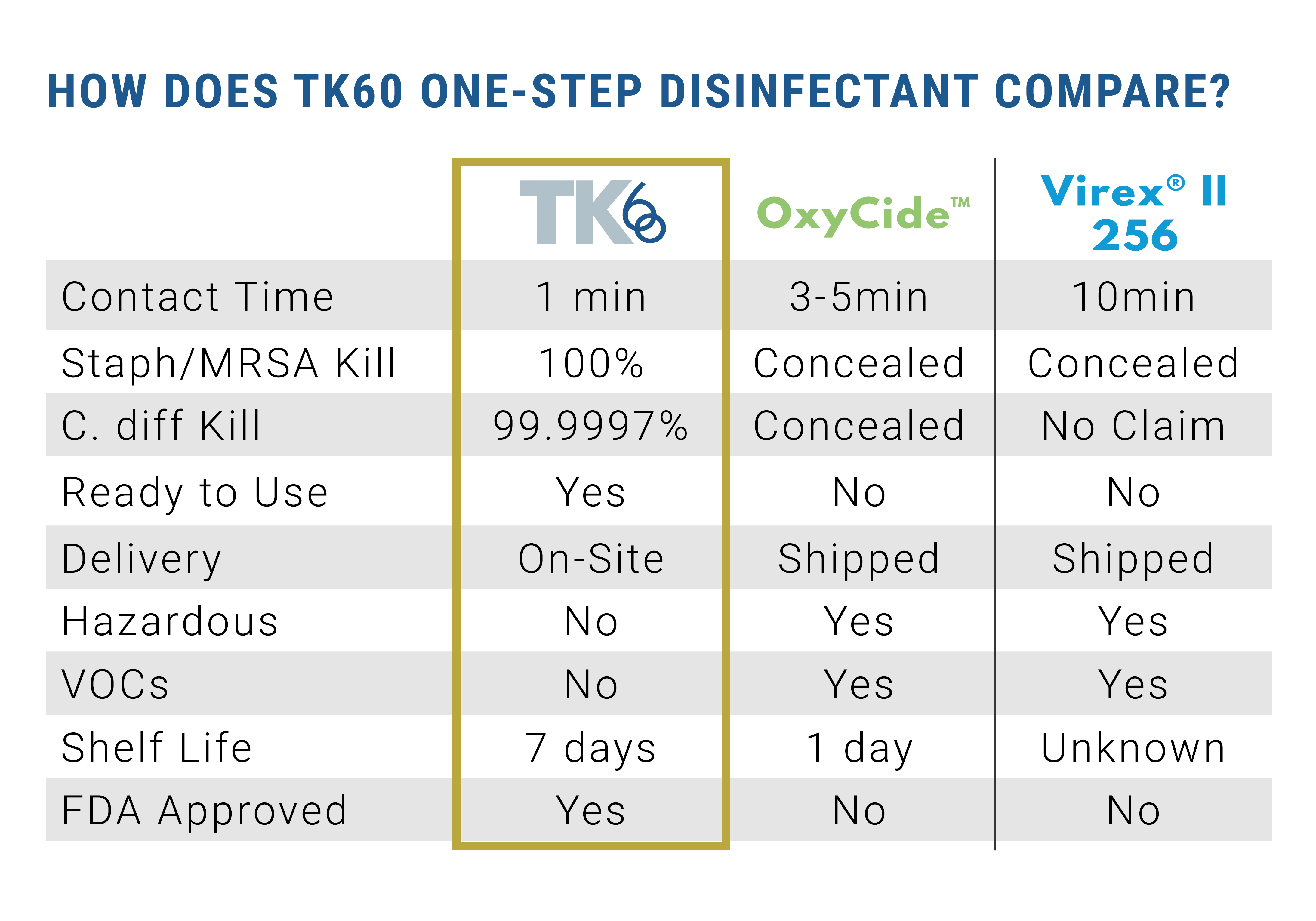
EPA lab testing proves TK60 kills HAI causing pathogens, such as C-diff and MRSA, in 60 seconds. This is substantially less than the three to 10 minute contact time needed for products currently on the market, dramatically decreasing the amount of time patients and healthcare workers are exposed to harmful pathogens and chemicals.
 The CDC recommends that, “Ideally, product users should consider and use products that have a shortened contact time.”
The CDC recommends that, “Ideally, product users should consider and use products that have a shortened contact time.”
Regarding products with a 10 minute contact time, the CDC states: “Such a long contact time is not practical for disinfection of environmental surfaces in a healthcare setting because most healthcare facilities apply a disinfectant and allow it to dry.”
Why is TK60 so effective?
TK60 is a water-based disinfectant, composed mainly of 99 percent water and .02 percent HOCl.
According to the CDC, “the microbicidal activity of chlorine is attributed largely to undissociated hypochlorous acid (HOCl). The dissociation of HOCI to the less microbicidal form, hypochlorite ion OCl¯, depends on pH. The disinfecting efficacy of chlorine decreases with an increase in pH.”
“EPA lab testing proves TK60 kills HAI causing pathogens, such as C-diff and MRSA, in 60 seconds”
Chlorine, at a pH of 12 commonly known as household bleach, is composed of the hypochlorite ion, and it is a relatively ineffective disinfectant when compared to HOCl.
The CDC adds: “The concept of electrolyzing saline to create a disinfectant or antiseptics is appealing because the basic materials of saline and electricity are inexpensive, and the end product, i.e. water, does not damage the environment.”
 TK60 is FDA approved to be used on food and food-contact surfaces, and it does not require an extra rinse to remove residue. TK60 is proven to be non-carcinogenic, complies with OSHA standards for use against blood borne pathogens, and resistant strains of bacteria cannot form against it. TK60 has a seven day shelf life in a closed container.
TK60 is FDA approved to be used on food and food-contact surfaces, and it does not require an extra rinse to remove residue. TK60 is proven to be non-carcinogenic, complies with OSHA standards for use against blood borne pathogens, and resistant strains of bacteria cannot form against it. TK60 has a seven day shelf life in a closed container.
FC+, also generated by R-Water’s device, produces soap when in contact with oils and animal fats in a process called saponification. FC+ is a dilute form of sodium hydroxide. The TC-RU produces FC+ at 540 ppm. At levels lower than 2000 ppm, sodium hydroxide, or FC+, is not considered a skin irritant. FC+ can be added to auto-scrubbers, carpet cleaning equipment, and mop buckets to be used for exceptional cleaning. FC+ will not leave a residue, helping to reduce the risk of slips and falls in facilities.
“Ideally, product users should consider and use products that have a shortened contact time”
Both solutions can be used safely on a variety of surfaces, and they do not contain allergy causing perfumes or dyes. TK60 and FC+ are not harmful to humans or animals, do not require personal protective equipment (PPE) when handling, and they can be safely discharged down drains. TK60 and FC+ are dispensed ready to use with no mixing or diluting necessary. On-site, ready-to- use production eliminates a facility’s need to store or mix hazardous chemicals and comply with the rigorous disposal practices OSHA and the EPA require of hazardous chemical containers.
 R-Water’s solutions have been tested, approved, and comply with EPA, FDA and OSHA standards. The TC-RU device has passed UL testing for safety, and R-Water’s manufacturing facility is audited quarterly by TUV for quality assurance.
R-Water’s solutions have been tested, approved, and comply with EPA, FDA and OSHA standards. The TC-RU device has passed UL testing for safety, and R-Water’s manufacturing facility is audited quarterly by TUV for quality assurance.
So, why isn’t every healthcare facility using R-Water’s device to reduce risk and improve safety for patients and workers, while benefitting from cost savings?
Many healthcare organizations have not updated their internal protocol to reflect this EPA Regulatory Classification of Devices.
True to their values, R-Water posts documentation to validate their claims on their website. For more information visit www.r-water.com.






Recent Comments